√ Stable current transmission, precise treatment
√ Durable and resistant to disinfection, suitable for clinical conditions
√ Clinically compatible, easy to use
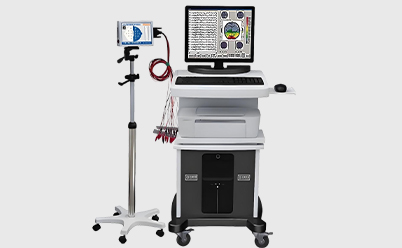
| Designed specifically for medical bone injury healing electrotherapy devices, its core function is to connect the main unit of the electrotherapy device to the surface electrode pads, enabling precise transmission of low-frequency pulse current (positive and negative polarity conduction). |
Designed specifically for medical bone injury healing electrotherapy devices, its core function is to connect the main unit of the electrotherapy device to the surface electrode pads, enabling precise transmission of low-frequency pulse current (positive and negative polarity conduction). It is suitable for orthopedic electrotherapy scenarios such as fracture healing, delayed bone healing, and osteoporosis. The wiring harness utilizes medical-grade biocompatible materials, a high-insulation protective structure, and low-impedance conductors. Combined with sterile anti-detachment connectors and orthopedic-specific accessories, it possesses characteristics such as disinfection resistance, bending resistance, stable current transmission, and biosafety. It can withstand the stringent requirements of repeated clinical use, various disinfection methods, and contact with the human body surface, making it a core connecting component ensuring the safety and effectiveness of bone injury electrotherapy.
|
Item
|
Specification Details
|
|
Applicable Scenarios
|
Electrotherapy scenarios such as fracture healing, delayed bone union, osteoporosis, and post-orthopedic surgery rehabilitation
|
|
Core Purpose
|
Transmission of low-frequency pulsed current (positive and negative poles) between electrotherapy device host and surface electrode pads
|
|
Compatible Equipment
|
Orthopedic healing electrotherapy devices from brands such as Ossur, DJI Medical, Kangfuzhijia, and Yuyue; Compatible with standard surface electrode pads
|
|
Operating Parameters
|
Treatment Current: 0~50mA; Pulse Frequency: 1~100Hz; Rated Voltage: DC 0~30V
|
|
Cable Specification
|
Positive/Negative Wires: 24AWG~20AWG Multi-strand Tinned Copper Conductor (Conductivity ≥98%)
|
|
Insulation/Sheath Material
|
Insulation Layer: PVC (High Insulation); Sheath: Medical-grade Silicone/PU (Biocompatibility Class VI)
|
|
Shielding Structure
|
Single-wire Independent Shielding (Foil + Tinned Copper Mesh, Coverage Rate ≥90%), reducing electromagnetic interference
|
|
Connector/Terminal Type
|
Medical-grade Sterile Anti-detachment Connectors, Quick-connect Foolproof Terminals (with positive/negative pole distinction); Contact Resistance <1mΩ
|
|
Protection Performance
|
Sterilization Resistant (Alcohol / PVP-I / EO Sterilization); Water-resistant IPX6 (Connectors); Resistant to human sweat corrosion
|
|
Safety Certifications
|
ISO 10993, GB 9706.1, CE MDR, RoHS, and relevant medical device registration certifications
|
|
Included Accessories
|
Sterile Packaging, Positive/Negative Pole Identification Stickers, Connector Protection Covers, Storage Straps, Sterilization Guide Card
|


If you are interested in any product, please contact us. We will introduce our products to you in more detail
